Educational aids
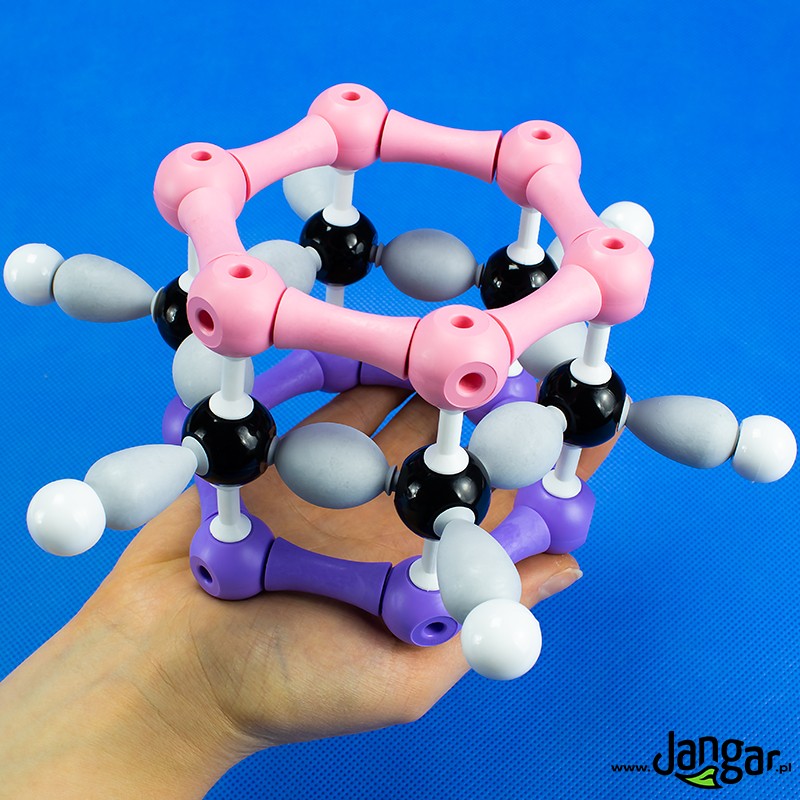
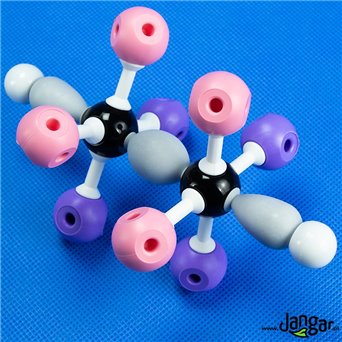
Model of benzene, ethane, ethene, ethin
Product reference: Che000100
Tax excl.: zł173.17
Tax incl.: zł213.00
Tax incl.: zł213.00
Description
MACHINE TRANSLATION (not authorised yet)
Set of 4 models: benzene, ethane, ethene, ethylene. This series contains enough parts to make 4 organic molecular orbitals models. The models show: sigma bonding orbitals, pi bonding orbitals, the concept of hybridization and delocalization.Kit includes:
H hydrogen atoms, 18 pcs,
carbon C atoms, 12 pcs.
sigma bonds, 27 pcs. (9 carbon-carbon bonds, 18 carbon-hydrogen bonds)
pi bonds, 9 units.
Worth knowing...
Pi (π) bond: is a chemical bond formed by the lateral superposition of atomic orbitals (ignoring s orbitals), and the molecular π orbital determines its shape.
Sigma bond (σ): it is a chemical bond formed by the frontal overlap of two atomic orbitals, thus creating two new orbitals: a bonding and an anti-bonding. The σ molecular orbital determines the shape of the σ bond.
Delocalized electron - a single electron involved in the formation of more than 1 bond. This situation usually occurs when the p orbitals (orbital) of multiple neighboring atoms in a molecule are close enough together to overlap equally.
Product Details
Che000100
You might also like